9.30.2010
9.27.2010
Water's Quantum Jazz Cooperative and Coherent Water
ISIS Report 27/09/10
##########################
Cooperative hydrogen-bonding between molecules gives rise to energetically
favourable three-dimensional network of supramolecular clusters in liquid water
under ambient conditions, resulting in long-range dipole correlation and quantum
coherence Dr. Mae-Wan Ho
Confessions of a hydrophiliac
As a self-confessed hydrophiliac, I love nothing more than to immerse myself in
water, literally and figuratively. I have been obsessed with water’s unfolding
biopic as though my own life depends on it, and most certainly so (see [1] Two-
States Water Explains All? SiS 32, and other articles in the long running New
Age of Water series). Water is the simplest, commonest chemical compound on
earth. Yet, it has the most complex properties and baffling ‘anomalies’ compared
to its neighbours in the periodic table of chemical compounds, without which
life as we know it would be impossible. Water has remained a mystery to
generations of the best scientists who have pitched their wits (and
sophisticated instruments) at water, only to have it slip gracefully through
their fingers.
Water is perfectly transparent and has no colour, except when its fine droplets
refract sunlight into the dazzling spectrum of the rainbow. Water has no form
other than that of the containing vessel, no sound, no movement, and little
resistance; except when coaxed by the gentle breeze into smiling ripples, or
tickled into undulating waves lapping like laughter. Or else when whipped up by
hurricanes into howling surges that hurl ships into the air, or heaved by
submarine earthquakes into rumbling tsunamis that deluge shore and land.
The dramatically different, infinitely varied moods of water are the stuff of
life itself, if not also great art. Indeed, a good scientific theory needs to
capture the art, to explain the long range cohesion coalescing massive volumes
into gigantic whirlpools, and at the same time bespeaks the endless diversity in
molecular structures that makes every snowflake a unique event in the history of
the universe (see [2] Crystal Clear – Messages from Water, SiS 15).
This latest episode brings together evidence, old and new, that confirms what
many of us had suspected: water is coherent, if not quantum coherent, and it is
that which accounts for all its life-giving properties. We begin with some
basics on which almost everyone agrees.
Water loves bonding
Figure 1 The water molecule with separated positive and negative charges
The water molecule is a permanent dipole in which positive and negative charges
are separated, with the two hydrogen atoms at the positive pole and the oxygen
atom at the negative pole (see Fig.1). Like other dipoles, water molecules can
stack together in dipole interactions with alternating positive and negative
poles next to each other. It can also engage in electrostatic interactions with
charged ions and other dipoles dissolved in it.
In addition, the water molecule likes to hydrogen-bond with one another (Fig.
2), and with molecules and ions dissolved in it. A hydrogen bond consists of
hydrogen shared between two electronegative atoms such as oxygen or nitrogen.
The compound or group that donates the hydrogen is the hydrogen donor, while and
compound or group that accepts the hydrogen is the hydrogen acceptor. Water is
both hydrogen donor and acceptor; it can donate two hydrogens and its oxygen can
accept two other hydrogens. The water molecule is generally represented as a
tetrahedron with four ‘arms’ – two hydrogen donors and two hydrogen acceptors -
pointing at the vertices. This tetrahedral structure is typical of ordinary ice,
where all the water molecules are cross-linked in a crystalline, hexagonal array
(see below).
Figure 2 Hydrogen-bonded water molecules
Decades of research has resulted in a near-consensus that water at ambient
temperatures and pressures exists as a dynamic network of supramolecular
clusters where a proportion of the molecules are linked together by ‘flickering’
hydrogen bonds, similar to those in ordinary ice. It is also widely acknowledged
that the hydrogen-bonded network of liquid water accounts for most, if not all
its anomalous properties. Beyond that, there is no agreement over the exact
proportion of molecules linked by tetrahedral ice-like bonds, the precise
structure and size of the clusters, how freely the molecules can move around,
and especially whether interactions are strictly local with nearest neighbour,
or much more global in extent.
Within the past decade, substantial evidence has emerged indicating that
cooperative interactions between molecules results in remarkably long-range
coherence in liquid water under ambient conditions.
First of all, water has an unusually high dielectric constant of ~78 at room
temperature, making it the most important polar solvent in chemistry and
biology, it also means it is easily polarised by an electric field. The
dielectric constant, or relative static permittivity, is a measure of the extent
to which it concentrates electrostatic lines of flux relative to a vacuum.
Researchers led by Manu Sharma at Princeton University, New Jersey, USA, have
shown by molecular dynamics simulations from first principles that the high
dielectric constant of water is due to two effects of the hydrogen bonds
contributing in almost equal measure [3]. The hydrogen bonding serves to align
the dipoles and at the same time, pull away positive and negative charges within
a molecule, enhancing the average molecular moment.
Read the rest of this report here
http://www.i-sis.org.uk/cooperativeCoherentWater.php
Or read other reports about water here
http://www.i-sis.org.uk/SO_water.php
======================================
##########################
Cooperative hydrogen-bonding between molecules gives rise to energetically
favourable three-dimensional network of supramolecular clusters in liquid water
under ambient conditions, resulting in long-range dipole correlation and quantum
coherence Dr. Mae-Wan Ho
Confessions of a hydrophiliac
As a self-confessed hydrophiliac, I love nothing more than to immerse myself in
water, literally and figuratively. I have been obsessed with water’s unfolding
biopic as though my own life depends on it, and most certainly so (see [1] Two-
States Water Explains All? SiS 32, and other articles in the long running New
Age of Water series). Water is the simplest, commonest chemical compound on
earth. Yet, it has the most complex properties and baffling ‘anomalies’ compared
to its neighbours in the periodic table of chemical compounds, without which
life as we know it would be impossible. Water has remained a mystery to
generations of the best scientists who have pitched their wits (and
sophisticated instruments) at water, only to have it slip gracefully through
their fingers.
Water is perfectly transparent and has no colour, except when its fine droplets
refract sunlight into the dazzling spectrum of the rainbow. Water has no form
other than that of the containing vessel, no sound, no movement, and little
resistance; except when coaxed by the gentle breeze into smiling ripples, or
tickled into undulating waves lapping like laughter. Or else when whipped up by
hurricanes into howling surges that hurl ships into the air, or heaved by
submarine earthquakes into rumbling tsunamis that deluge shore and land.
The dramatically different, infinitely varied moods of water are the stuff of
life itself, if not also great art. Indeed, a good scientific theory needs to
capture the art, to explain the long range cohesion coalescing massive volumes
into gigantic whirlpools, and at the same time bespeaks the endless diversity in
molecular structures that makes every snowflake a unique event in the history of
the universe (see [2] Crystal Clear – Messages from Water, SiS 15).
This latest episode brings together evidence, old and new, that confirms what
many of us had suspected: water is coherent, if not quantum coherent, and it is
that which accounts for all its life-giving properties. We begin with some
basics on which almost everyone agrees.
Water loves bonding
Figure 1 The water molecule with separated positive and negative charges
The water molecule is a permanent dipole in which positive and negative charges
are separated, with the two hydrogen atoms at the positive pole and the oxygen
atom at the negative pole (see Fig.1). Like other dipoles, water molecules can
stack together in dipole interactions with alternating positive and negative
poles next to each other. It can also engage in electrostatic interactions with
charged ions and other dipoles dissolved in it.
In addition, the water molecule likes to hydrogen-bond with one another (Fig.
2), and with molecules and ions dissolved in it. A hydrogen bond consists of
hydrogen shared between two electronegative atoms such as oxygen or nitrogen.
The compound or group that donates the hydrogen is the hydrogen donor, while and
compound or group that accepts the hydrogen is the hydrogen acceptor. Water is
both hydrogen donor and acceptor; it can donate two hydrogens and its oxygen can
accept two other hydrogens. The water molecule is generally represented as a
tetrahedron with four ‘arms’ – two hydrogen donors and two hydrogen acceptors -
pointing at the vertices. This tetrahedral structure is typical of ordinary ice,
where all the water molecules are cross-linked in a crystalline, hexagonal array
(see below).
Figure 2 Hydrogen-bonded water molecules
Decades of research has resulted in a near-consensus that water at ambient
temperatures and pressures exists as a dynamic network of supramolecular
clusters where a proportion of the molecules are linked together by ‘flickering’
hydrogen bonds, similar to those in ordinary ice. It is also widely acknowledged
that the hydrogen-bonded network of liquid water accounts for most, if not all
its anomalous properties. Beyond that, there is no agreement over the exact
proportion of molecules linked by tetrahedral ice-like bonds, the precise
structure and size of the clusters, how freely the molecules can move around,
and especially whether interactions are strictly local with nearest neighbour,
or much more global in extent.
Within the past decade, substantial evidence has emerged indicating that
cooperative interactions between molecules results in remarkably long-range
coherence in liquid water under ambient conditions.
First of all, water has an unusually high dielectric constant of ~78 at room
temperature, making it the most important polar solvent in chemistry and
biology, it also means it is easily polarised by an electric field. The
dielectric constant, or relative static permittivity, is a measure of the extent
to which it concentrates electrostatic lines of flux relative to a vacuum.
Researchers led by Manu Sharma at Princeton University, New Jersey, USA, have
shown by molecular dynamics simulations from first principles that the high
dielectric constant of water is due to two effects of the hydrogen bonds
contributing in almost equal measure [3]. The hydrogen bonding serves to align
the dipoles and at the same time, pull away positive and negative charges within
a molecule, enhancing the average molecular moment.
Read the rest of this report here
http://www.i-sis.org.uk/cooperativeCoherentWater.php
Or read other reports about water here
http://www.i-sis.org.uk/SO_water.php
======================================
Subscribe to:
Comments (Atom)
Simple Schematic for Scooters/ Motor Bikes
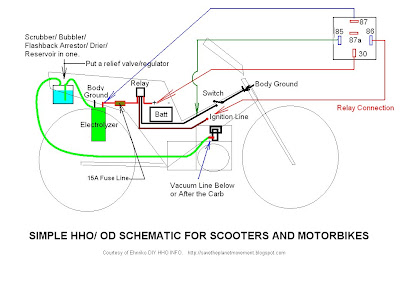
This is a very basic schematic for bikes.
HHO Chit Chat

Custom Search