This vid was long overdue.. for the novice to the advanced.. this is some of the basics describing why HHO is ENTIRELY DIFFERENT from Brown's gas ie; particulate water vapor from the re-mixing of gas w/solution substrate(then dried or not)... i'll point out that The Hydroxy Gas has been Photonated after an induced VOLTAGE Dissociation Field and "starved" of electrons.. this effect inhibits the Constituent Gasses from returning to a water state upon combustion.....KaaaaBOOOM!!!
This is a first rate vid..."theenergyunderground" has done their homework......
9.22.2009
SECRETS OF STANLEY MEYER'S WFC - Lecture 1 1
9.15.2009
Electrolysis

Electrolysis is a process by which electrical energy is used to produce a chemical change. Perhaps the most familiar example of electrolysis is the decomposition (breakdown) of water into hydrogen and oxygen by means of an electric current. The same process can be used to decompose compounds other than water. Sodium, chlorine, magnesium, and aluminum are four elements produced commercially by electrolysis.
Principles
The electrolysis of water illustrates the changes that take place when an electric current passes through a chemical compound. Water consists of water molecules, represented by the formula H 2 O. In any sample of water, some small fraction of molecules exist in the form of ions, or charged particles. Ions are formed in water when water molecules break apart to form positively charged hydrogen ions and negatively charged hydroxide ions. Chemists describe that process with the following chemical equation:
H 2 O → H + + OH −
In order for electrolysis to occur, ions must exist. Seawater can be electrolyzed, for example, because it contains many positively charged sodium ions (Na + ) and negatively charged chloride ions (Cl − ). Any liquid, like seawater, that contains ions is called an electrolyte.
Water is not usually considered an electrolyte because it contains so few hydrogen and hydroxide ions. Normally, only one water molecule out of two billion ionizes. In contrast, sodium chloride (table salt) breaks apart completely when dissolved in water. A salt water solution consists entirely of sodium ions and chloride ions.
In order to electrolyze water, then, one prior step is necessary. Some substance, similar to sodium chloride, must be added to water to make it an electrolyte. The substance that is usually used is sulfuric acid.
The electrolysis process
The equipment used for electrolysis of a compound consists of three parts: a source of DC (direct) current; two electrodes; and an electrolyte. A common arrangement consists of a battery (the source of current) whose two poles are attached to two strips of platinum metal (the electrodes), which are immersed in water to which a few drops of sulfuric acid have been added (the electrolyte).
Electrolysis begins when electrical current (a flow of electrons) flows out of one pole of the battery into one electrode, the cathode. Positive hydrogen ions (H + ) in the electrolyte pick up electrons from that electrode and become neutral hydrogen molecules (H 2 ):
2 H + + 2 e − → H 2
(Hydrogen molecules are written as H 2 because they always occur as pairs of hydrogen atoms. The same is true for molecules of oxygen, O 2 .)
As the electrolysis of water occurs, one can see tiny bubbles escaping from the electrolyte at the cathode. These are bubbles of hydrogen gas.
Words to Know
Anode: The electrode in an electrolytic cell through which electrons move from the electrolyte to the battery.
Cathode: The electrode in an electrolytic cell through which electrons move from the battery to the electrolyte.
Electrolyte: Any substance that, when dissolved in water, conducts an electric current.
Electrolytic cell: A system in which electrical energy is used to bring about chemical changes.
Electroplating: A process that uses an electrolytic cell to deposit a thin layer of metal on some kind of surface.
Ion: Any particle, such as an atom or molecule, that carries an electric charge.
Bubbles can also be seen escaping from the second electrode, the anode. The anode is connected to the second pole of the battery, the pole through which electrons enter the battery. At this electrode, electrons are being taken out of the electrolyte and fed back into the battery. The electrons come from negatively charged hydroxide ions (OH − ), which have an excess of electrons. The anode reaction is slightly more complicated than the cathode reaction, as shown by this chemical equation:
4 OH − − 4 e − → O 2 + 2 H 2 O
Essentially this equation says that electrons are taken away from hydroxide ions and oxygen gas is produced in the reaction. The oxygen gas bubbles off at the anode, while the extra water formed remains behind in the electrolyte.
The overall reaction that takes place in the electrolysis of water is now obvious. Electrons from the battery are given to hydrogen ions in the electrolyte, changing them into hydrogen gas. Electrons are taken from hydroxide ions in the electrolyte and transferred to the battery. Over time, water molecules are broken down to form hydrogen and oxygen molecules:
2 H 2 O → 2 H 2 + O 2
Commercial applications
Preparing elements. Electrolysis is used to break down compounds that are very stable. For example, aluminum is a very important metal in modern society. It is used in everything from pots and pans to space shuttles. But the main natural source of aluminum, aluminum oxide, is a very stable compound. A compound that is stable is difficult to break apart. You can't get aluminum out of aluminum oxide just by heating the compound—you need more energy than heat can provide.
Aluminum is prepared by an electrolytic process first discovered in 1886 by a 21-year-old student at Oberlin College in Ohio, Charles Martin Hall (1863–1914). Hall found a way of melting aluminum oxide and then electrolyzing it. Once melted, aluminum oxide forms ions of aluminum and oxygen, which behave in much the same way as hydrogen and hydroxide ions in the previous example. Pure aluminum metal is obtained at the cathode, while oxygen gas bubbles off at the anode. Sodium, chlorine, and magnesium are three other elements obtained commercially by an electrolytic process similar to the Hall process.
Refining of copper. Electrolysis can be used for purposes other than preparing elements. One example is the refining of copper. Very pure copper is often required in the manufacture of electrical equipment. (A purity of 99.999 percent is not unusual.) The easiest way to produce a product of this purity is with electrolysis.
An electrolytic cell for refining copper contains very pure copper at the cathode, impure copper at the anode, and copper sulfate as the electrolyte. When the anode and cathode are connected to a battery, electrons flow into the cathode, where they combine with copper ions (Cu 2+ ) in the electrolyte:
Cu 2+ + 2 e − → Cu 0
Pure copper metal (Cu 0 in the above equation) is formed on the cathode.
At the anode, copper atoms (Cu 0 ) lose electrons and become copper ions (Cu 2+ ) in the electrolyte:
Cu 0 − 2 e − → Cu 2+
Overall, the only change that occurs in the cell is that copper atoms from the impure anode become copper ions in the electrolyte. Those copper ions are then plated out on the cathode. Any impurities in the anode are just left behind, and nearly 100 percent pure copper builds up on the cathode.
Electroplating. Another important use of electrolytic cells is in the electroplating of silver, gold, chromium, and nickel. Electroplating produces a very thin coating of these expensive metals on the surfaces of cheaper metals, giving them the appearance and the chemical resistance of the expensive ones.
In silver plating, the object to be plated (a spoon, for example) is used as the cathode. A bar of silver metal is used as the anode. And the electrolyte is a solution of silver cyanide (AgCN). When this arrangement is connected to a battery, electrons flow into the cathode where they combine with silver ions (Ag + ) from the electrolyte to form silver atoms (Ag 0 ):
Ag + + 1 e − → Ag 0
These silver atoms plate out as a thin coating on the cathode—in this case, the spoon. At the anode, silver atoms give up electrons and become silver ions in the electrolyte:
Ag 0 − 1 e − → Ag 0
Silver is cycled, therefore, from the anode to the electrolyte to the cathode, where it is plated out.
Read more: http://www.scienceclarified.com/El-Ex/Electrolysis.html#ixzz0RFoButTG
Subscribe to:
Comments (Atom)
Simple Schematic for Scooters/ Motor Bikes
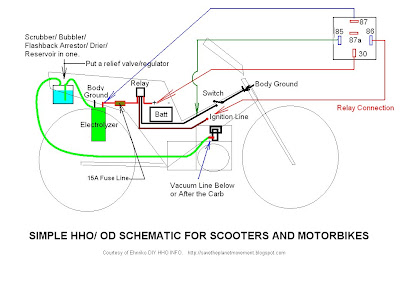
This is a very basic schematic for bikes.
HHO Chit Chat

Custom Search